Rarely, the thoracic duct is damaged during thoracic surgery, and can be detected as a left-sided chylous pleural effusion (chylopericardium has also been reported). The traditional treatment for thoracic duct injury with left chylothorax has been clipping the thoracic duct followed by pleurodesis, but in 1998 percutaneous access of the thoracic duct was first attempted, and a 45-71% success rate of percutaneous embolization has lead some to try it as a first pass to avoid surgery.
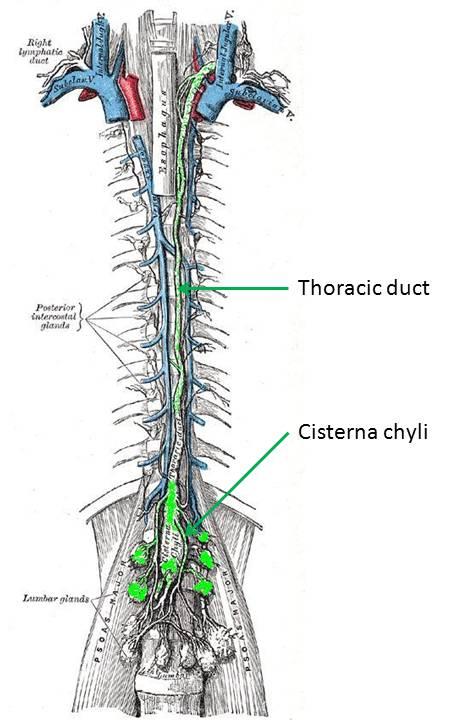 Transection of the thoracic duct is not the only way to cause a
chylothorax, however. It has been noted that compression or stenosis of
the thoracic duct leads to the development of small, fragile
collaterals running in parallel. These thin collaterals are easily
damaged and can rupture into the pleural space.
Transection of the thoracic duct is not the only way to cause a
chylothorax, however. It has been noted that compression or stenosis of
the thoracic duct leads to the development of small, fragile
collaterals running in parallel. These thin collaterals are easily
damaged and can rupture into the pleural space.First, a little bit about the thoracic duct... The lymphatics begin as end-bulbs (lacteals in the intestine) and merge into progressively larger lymphatic channels. The lymph moves centrally due to the action of one-way valves, spontaneous contraction of lymphatic channels, and muscular or organ activity. These channels eventually converge around the L1-L2 vertebra. If the confluence is centralized and fusiform, it's termed a cisterna chyli. The thoracic duct arises from this level and rises between the esophagus and aorta, to empty into the left internal jugular or left subclavian vein... thus returning the lymph to the vascular blood stream. The thoracic duct can have multiple or duplicated channels within it. Lymph at the periphery is clear and colorless, but lymph originating at the intestine (from the lacteals) is often cloudy since it is a suspension containing fat (about 60-70% of fat makes its way to the bloodstream through the lymphatic system).
So how do you access the thoracic duct? Well... that's the trick. It would seem tempting to try to cannulate it in a retrograde manner through its cephalad venous confluence, but this is not only very technically difficult, but if there is a complete transection of the skinny thoracic duct, then you also won't be able to embolize the outflow channel.
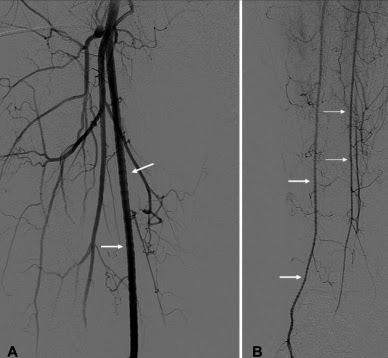
So instead, you have to go antegrade... but how do you find the upstream entrance to the thoracic duct? The trick is to perform a pedal lymphangiogram first as a road map. First lymphazurin is injected into the subcutaneous soft tissues (at the interdigital spaces of the first, second, and third toes). A small incision is then made and the colored lymphazurin ("lymph" + "azure") highlights the foot lymphatic channels in the subcutaneous tissues. A lymphatic channel is then selected and cannulated with a 30 gauge catheter, after which lipiodol is slowly infused (below). Multiple lymphatic channels can be cannulated to try to increase the amount of contrast in the lymphatic system.
 |
| Lipiodol contrast extending up the lymphatics of the lower extremity toward the pelvis, and from there to the abdomen and cisterna chyli. |




.JPG)










.jpg)