Transient Ischemic Dilatation (TID) is an important finding on myocardial perfusion SPECT in that it can be an indication of extensive severe coronary artery disease. The reversible/transient nature of the dilatation corresponds to its ischemic nature, and TID has been associated with a greater risk of future cardiac events. In one study, TID was associated with proximal LAD or multivessel >90% stenosis. In another study a transient dilatation ratio of 1.12 was 60% sensitive and 95% specific for critical multivessel CAD. Despite this, however, these findings are associated with a defect in the myocardial perfusion with stress; it is unclear how severe CAD really is in a patient with TID and no
abnormality in myocardial perfusion.
Tuesday, December 11, 2012
Monday, December 10, 2012
Pulsed Wave Doppler and Aliasing
Pulsed Doppler is in distinction to continuous wave Doppler.
In continuous wave Doppler, reflectors anywhere in the ultrasound transducer beam contribute to the Doppler signal.
In pulsed Doppler, a piece of the return signal can be selected, thereby only detecting the moving particles at a certain depth and in a particular volume (the "sample volume"). Even though the volume cannot be appreciated on 2D grayscale imaging, it can lead to localization artifact (Doppler signal can be acquired from nearby vessels, not necessarily in the field of view).
In continuous wave Doppler, reflectors anywhere in the ultrasound transducer beam contribute to the Doppler signal.
 |
| From Ref 2 |
In pulsed Doppler, a piece of the return signal can be selected, thereby only detecting the moving particles at a certain depth and in a particular volume (the "sample volume"). Even though the volume cannot be appreciated on 2D grayscale imaging, it can lead to localization artifact (Doppler signal can be acquired from nearby vessels, not necessarily in the field of view).
Sunday, December 9, 2012
The Doppler Equation
Doppler ultrasound interprets the frequency as well as the amplitude of returning sound waves. The Doppler frequency (fD) is defined as the difference between the received and transmitted frequencies of the moving sound wave scatterers (RBCs).
The Doppler angle strongly influences the amount of return signal, with a maximum parallel to the flow (cos 0 = 1), and no signal perpendicular to the flow (cos 90 = 0). The return signal is half strength at 60 degrees (cos 60 =0.5)
---
1. "Introduction to Vascular Ultrasonography" Zweibel and Pellerito, ed. 5th ed. (2005).
 |
| The Doppler equation: f0, initial sound frequency; fr, return sound frequency; v, flow velocity; c, speed of sound. |
The Doppler angle strongly influences the amount of return signal, with a maximum parallel to the flow (cos 0 = 1), and no signal perpendicular to the flow (cos 90 = 0). The return signal is half strength at 60 degrees (cos 60 =0.5)
---
1. "Introduction to Vascular Ultrasonography" Zweibel and Pellerito, ed. 5th ed. (2005).
Saturday, December 8, 2012
Fluoroscopic Landmarks in Femoral Artery Puncture
Although needle access to the femoral artery is most simply performed with palpation, in situations where this is limited (e.g. weak pulses due to atherosclerotic disease? obesity?), ultrasound could easily be used for guidance.
In the old days before readily available high-frequency ultrasound, however, fluoroscopic landmarks were used to help guide puncture in more challenging situations. The most stable landmark is the relationship of the common femoral artery overlying the medial femoral head (below). In one study, the center line of the common femoral artery projected over the medial half of the femoral head in 93% of patients (medial third in 69%, medial to the femoral head in 6%). One source suggests an initial puncture 1 cm lateral to the most medial cortex of the femoral head as the best site for both retrograde and anterograde femoral artery puncture.
This landmark is also useful in that it is above the femoral artery bifurcation and below the inguinal ligament. Puncture of the external iliac artery above the inguinal ligament is associated with significantly higher bleeding complications. Distal SFA punctures are associated with a greater risk of dissection and thrombosis, increased risk of hematoma and pseudoaneurysm, and an increased risk of AVF. A puncture over the femoral head also aids in compression and cessation of bleeding after removing the sheath.
Other points to remember are that calcifications in the arteries can be used to help determine where the lumen is, and that the landmarks mentioned above are critically dependent on normal AP orientation, and deviation from the normal AP orientation can result in a large change in position of the artery relative to the femoral head.
The flip side of recognizing fluoroscopic landmarks is to avoid the vascular structure when making a puncture to access the hip joint space.
Ultrasound guidance is probably prudent in anticoagulated patients with a difficult access approach (obesity, scar, hematoma). The vessel should be punctured as centrally as possible.
---
1. Dotter CT, Rosch J, Robinson M. "Fluoroscopic Guidance in Femoral Artery Puncture" Radiology 127:266-267, April 1978
2. Wacker F, Wolf KJ, Fobbe F. "Percutaneous vascular access guided by color duplex sonography" Eur Radiol. 1997;7:1501-1504.
3. Rutherford's Vascular Surgery. Cronenwett and Johnston. 7th ed. (2010)
In the old days before readily available high-frequency ultrasound, however, fluoroscopic landmarks were used to help guide puncture in more challenging situations. The most stable landmark is the relationship of the common femoral artery overlying the medial femoral head (below). In one study, the center line of the common femoral artery projected over the medial half of the femoral head in 93% of patients (medial third in 69%, medial to the femoral head in 6%). One source suggests an initial puncture 1 cm lateral to the most medial cortex of the femoral head as the best site for both retrograde and anterograde femoral artery puncture.
This landmark is also useful in that it is above the femoral artery bifurcation and below the inguinal ligament. Puncture of the external iliac artery above the inguinal ligament is associated with significantly higher bleeding complications. Distal SFA punctures are associated with a greater risk of dissection and thrombosis, increased risk of hematoma and pseudoaneurysm, and an increased risk of AVF. A puncture over the femoral head also aids in compression and cessation of bleeding after removing the sheath.
Other points to remember are that calcifications in the arteries can be used to help determine where the lumen is, and that the landmarks mentioned above are critically dependent on normal AP orientation, and deviation from the normal AP orientation can result in a large change in position of the artery relative to the femoral head.
The flip side of recognizing fluoroscopic landmarks is to avoid the vascular structure when making a puncture to access the hip joint space.
Ultrasound guidance is probably prudent in anticoagulated patients with a difficult access approach (obesity, scar, hematoma). The vessel should be punctured as centrally as possible.
---
1. Dotter CT, Rosch J, Robinson M. "Fluoroscopic Guidance in Femoral Artery Puncture" Radiology 127:266-267, April 1978
2. Wacker F, Wolf KJ, Fobbe F. "Percutaneous vascular access guided by color duplex sonography" Eur Radiol. 1997;7:1501-1504.
3. Rutherford's Vascular Surgery. Cronenwett and Johnston. 7th ed. (2010)
Friday, December 7, 2012
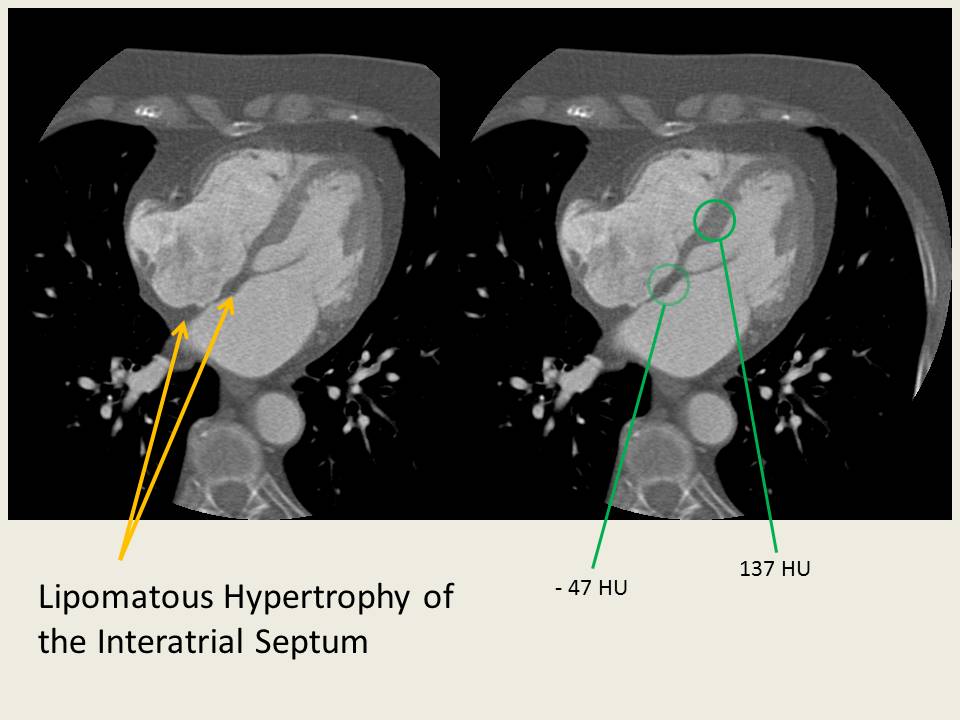
Lipomatous Hypertrophy of the Interatrial Septum
An occasional incidental finding on Cardiac CT and MR (and transesophageal echocardiography) is Lipomatous Hypertrophy of the Interatrial Septum.
Monday, November 26, 2012
Hepatic Artery Complications Following Liver Transplant
After a liver transplant, the anastomosed hepatic artery, portal vein, and hepatic veins/IVC can all be a source of problems. Of the three systems, the hepatic artery connection(s) tends to be the most common offender, particularly with stenosis and thrombosis at the anastomosis site. The incidence of hepatic anastomosis complications has been reported at somewhere between 4-25%.
Problems are usually detected on routine Doppler ultrasound of the hepatic vasculature.
The hepatic artery normally demonstrates a low-resistance waveform with continuous forward flow during diastole. The resistive index is somewhere between 0.5 - 0.7. With stenosis, there is a focal area of increased flow with post-stenotic aliasing, and, as with stenoses elsewhere, there can be a downstream tardus parvus waveform.
If Doppler ultrasound detects a problem, the area can be further investigated with angiography. In the two examples below, the high-grade stenosis turned out to be asymptomatic and not to be flow-limiting, so therapy was deferred... however stenosis can lead to graft dysfunction, bilary leak (transplant bile ducts receive all their blood supply from the transplant hepatic artery), or frank hepatic necrosis.
Problems are usually detected on routine Doppler ultrasound of the hepatic vasculature.
The hepatic artery normally demonstrates a low-resistance waveform with continuous forward flow during diastole. The resistive index is somewhere between 0.5 - 0.7. With stenosis, there is a focal area of increased flow with post-stenotic aliasing, and, as with stenoses elsewhere, there can be a downstream tardus parvus waveform.
 |
| Normal hepatic low-resistance arterial flow (from Ref 3) |
If Doppler ultrasound detects a problem, the area can be further investigated with angiography. In the two examples below, the high-grade stenosis turned out to be asymptomatic and not to be flow-limiting, so therapy was deferred... however stenosis can lead to graft dysfunction, bilary leak (transplant bile ducts receive all their blood supply from the transplant hepatic artery), or frank hepatic necrosis.
 |
| High-grade stenosis at the hepatic arterial anastomosis. |
Friday, November 23, 2012
Aorto-enteric fistula
There are a number of serious complications of surgical aorta repair, one of which is... aorto-enteric fistula (AEF). Patients can present with massive GI bleeding (hematemesis, melena/hematochezia), sepsis/fever, and abdominal pain... and the prognosis is unfavorable.
Aortoenteric fistula usually occurs at the proximal anastomosis (region of infrarenal aorta and 3rd/4th part of the duodenum) and can result in massive bleeding into the bowel. Although AEF is usually separated from "perigraft infection" in terms of classification, the graft is invariably infected in aorto-enteric fistula (backflow from enteric contents). Infection of the graft causing the AEF is also considered a significant etiology (40% of infected grafts become AEFs) as prosthetic material can serve as a nidus for bacterial growth. Some feel that infection may play a role in all AEFs, but many may arise from lower-grade infections.
Graft-enteric fistulas can potentially occur anywhere along the graft, although they tend to occur more proximally. Of course, they're more common at weak points such anastomotic suture lines, but a fistula can occur even with native aneurysms (considered a primary aorto-enteric fistula).
So what are the findings with an aorto-enteric fistula? If upper GI bleeding is the provisional diagnosis and the patient is stable, EGD is often used as a first line modality. But if it's not suspected / or if the EGD is not definitive, then the patient may go to CT. As the name AEF implies, you look for an exchange of contents between the aorta and the bowel. Gas into the area area around the graft, and blood into the adjacent bowel. Actual visualization of extravasation is rare.
Aortoenteric fistula usually occurs at the proximal anastomosis (region of infrarenal aorta and 3rd/4th part of the duodenum) and can result in massive bleeding into the bowel. Although AEF is usually separated from "perigraft infection" in terms of classification, the graft is invariably infected in aorto-enteric fistula (backflow from enteric contents). Infection of the graft causing the AEF is also considered a significant etiology (40% of infected grafts become AEFs) as prosthetic material can serve as a nidus for bacterial growth. Some feel that infection may play a role in all AEFs, but many may arise from lower-grade infections.
Graft-enteric fistulas can potentially occur anywhere along the graft, although they tend to occur more proximally. Of course, they're more common at weak points such anastomotic suture lines, but a fistula can occur even with native aneurysms (considered a primary aorto-enteric fistula).
So what are the findings with an aorto-enteric fistula? If upper GI bleeding is the provisional diagnosis and the patient is stable, EGD is often used as a first line modality. But if it's not suspected / or if the EGD is not definitive, then the patient may go to CT. As the name AEF implies, you look for an exchange of contents between the aorta and the bowel. Gas into the area area around the graft, and blood into the adjacent bowel. Actual visualization of extravasation is rare.
Wednesday, November 14, 2012
The Vasculitides: Wegener Granulomatosis (WG)
Like Churg-Strauss and Microscopic Polyangiitis, these can attack both the lungs and the kidneys (Goodpasture's syndrome is another condition with pulmonary-renal involvement... but it's not a vasculitis). Wegener Granulomatosis is actually associated with a triad
1) Upper airway involvement (e.g. sinusitis, tracheobronchial thickening)
2) Lower airway involvment
3) Renal involvment (glomerulonephritis)
(even though the triad is helpful, it's not complete, since WG has been documented to attack just about every system in the body)
Unlike Churg-Strauss and Microscopic Polyangiitis, however, Wegener Granulomatosis has certain radiologic features that are relatively characteristic to it.
The first of these is the characteristic cavitating pulmonary nodules in Wegener Granulomatosis. Although WG can present with a wide variety of nonspecific pulmonary findings, the cavitating nodules are characteristic and "pulmonary nodules or masses" are present in 70% of patients at some point in the disease.
 |
| Multiple pulmonary nodules in a first presentation of bx-proven Wegner Granulomatosis. The groundglass halo is compatible with perinodular capillaritis and alveolar hemorrhage. |
Tuesday, November 13, 2012
The Vasculitides: Henoch-Schonlein Purpura (HSP)
When Henoch-Scholein Purpura (HSP) comes up, the association most quickly made is that of a child with erythematous papules and palpable purpura...
... but the source of the palpable purpura is a small-vessel vasculitis, and like other vasculitides, HSP is a multisystem disorder, affecting the joints, kidneys, and GI tract. The official 1990 ACR criteria are 2+/4 of:
Like in microscopic polyangiitis (see yesterday's post) the glomerulonephritis from the small-vessel vasculitis is actually the most serious aspect of HSP, but it's not well-appreciated radiologically.
GI manifestations of HSP, however, can be picked up... and since it's estimated that 65-75% of people with HSP have GI symptoms (colicky abdominal pain, vomiting, bloody stools), the radiological findings in HSP are characteristic enough to help determine if a patient's acute abdomen is related to the vasculitis or if it has some other source.
One way to think about the GI involvement of HSP is that what it's doing to the skin... it's also doing to the bowel. The abdominal pain in HSP is related to visceral purpura leading to submucosal and mucosal extravasation of blood and edema, which can lead to ulceration of the bowel mucosa. this can occur anywhere from the duodenum to the colon, and can often be seen with endoscopy.
On fluoroscopy, these changes result in thickening of the bowel wall, with thickened folds, thumbprinting, separation of loops, and prolonged transit time. Infrequently (1-5%) the bowel wall thickening can act as a lead point for intussusception. This appearance is not specific for HSP, however, and in older patients, other more common diagnoses, such as Crohns disease or infection should be entertained. Lupus causing small artery vasculitis should also be entertained.
... but the source of the palpable purpura is a small-vessel vasculitis, and like other vasculitides, HSP is a multisystem disorder, affecting the joints, kidneys, and GI tract. The official 1990 ACR criteria are 2+/4 of:
1. Palpable purpura
2. Age younger than 20y at disease onset
3. Bowel angina
4. Biopsy showing granulocytes in the wall of arterioles or venules.
Like in microscopic polyangiitis (see yesterday's post) the glomerulonephritis from the small-vessel vasculitis is actually the most serious aspect of HSP, but it's not well-appreciated radiologically.
GI manifestations of HSP, however, can be picked up... and since it's estimated that 65-75% of people with HSP have GI symptoms (colicky abdominal pain, vomiting, bloody stools), the radiological findings in HSP are characteristic enough to help determine if a patient's acute abdomen is related to the vasculitis or if it has some other source.
One way to think about the GI involvement of HSP is that what it's doing to the skin... it's also doing to the bowel. The abdominal pain in HSP is related to visceral purpura leading to submucosal and mucosal extravasation of blood and edema, which can lead to ulceration of the bowel mucosa. this can occur anywhere from the duodenum to the colon, and can often be seen with endoscopy.
On fluoroscopy, these changes result in thickening of the bowel wall, with thickened folds, thumbprinting, separation of loops, and prolonged transit time. Infrequently (1-5%) the bowel wall thickening can act as a lead point for intussusception. This appearance is not specific for HSP, however, and in older patients, other more common diagnoses, such as Crohns disease or infection should be entertained. Lupus causing small artery vasculitis should also be entertained.
Monday, November 12, 2012
The Vasculitides: Microscopic Polyangiitis (MPA)
There are no pictures in this post... because there is no classic radiographic appearance of microscopic polyangiitis (MPA). Just as its generic name implies, it has a nonspecific presentation...
An ANCA+ small vessel vasculitis in the group of Wegener's granulomatosis and perhaps Churg-Strauss syndrome, microscopic polyangiitis is being frequently "isolated" out from prior studies in which it was lumped in with other, more well-recognized vasculitides... for instance, it's been recognized that in some of the published series investigating polyarteritis nodosa, some of the cases were actually microscopic polyangiitis. They have many of the same clinical symptoms.
So how could you make a prospective radiologic call of microscopic polyangiitis? It would seem that you can't.
But you can choose not to suggest it when certain features are present.
1) As the name implies, it's a microscopic vasculitis, affecting capillaries, arterioles, and venules. Findings of medium or large vessel vasculitis on angiography do not occur.
2) Polyarteritis nodosa never involves the lungs. If you have findings suspicious for PAN, but with lung involvement (alveolar hemorrhage, diffuse alveolar damage), then microscopic polyangiitis is reasonable to put on the differential in its place.
3) Polyarteritis nodosa does not cause rapidly-progressive glomerulonephritis.
4) Microscopic polyangiitis does not cause renal aneurysms.
The age of presentation of MPA (avg. 50Y), is not significantly different than in other vasculitides. The renal-pulmonary presentation is similar to Goodpasture's syndrome. Occasionally, it can affect the GI tract, similar to other small vessel vasculitides, such as Henoch-Schonlein purpura.
---
1. "Vasculitis" Ball GV and Bridges SL. Oxford publishers (2002)
2. Ha HK, Lee SH, Rha SE. "Radiologic Features of Vasculitis Involving the Gastrointestinal Tract" RadioGraphics 2000; 20:779–794
An ANCA+ small vessel vasculitis in the group of Wegener's granulomatosis and perhaps Churg-Strauss syndrome, microscopic polyangiitis is being frequently "isolated" out from prior studies in which it was lumped in with other, more well-recognized vasculitides... for instance, it's been recognized that in some of the published series investigating polyarteritis nodosa, some of the cases were actually microscopic polyangiitis. They have many of the same clinical symptoms.
So how could you make a prospective radiologic call of microscopic polyangiitis? It would seem that you can't.
But you can choose not to suggest it when certain features are present.
1) As the name implies, it's a microscopic vasculitis, affecting capillaries, arterioles, and venules. Findings of medium or large vessel vasculitis on angiography do not occur.
2) Polyarteritis nodosa never involves the lungs. If you have findings suspicious for PAN, but with lung involvement (alveolar hemorrhage, diffuse alveolar damage), then microscopic polyangiitis is reasonable to put on the differential in its place.
3) Polyarteritis nodosa does not cause rapidly-progressive glomerulonephritis.
4) Microscopic polyangiitis does not cause renal aneurysms.
The age of presentation of MPA (avg. 50Y), is not significantly different than in other vasculitides. The renal-pulmonary presentation is similar to Goodpasture's syndrome. Occasionally, it can affect the GI tract, similar to other small vessel vasculitides, such as Henoch-Schonlein purpura.
---
1. "Vasculitis" Ball GV and Bridges SL. Oxford publishers (2002)
2. Ha HK, Lee SH, Rha SE. "Radiologic Features of Vasculitis Involving the Gastrointestinal Tract" RadioGraphics 2000; 20:779–794
Sunday, November 11, 2012
The Vasculitides: Churg-Strauss Syndrome (CSS)
Churg-Strauss syndrome is a small vessel vasculitis that represents a combined allergic granulomatosis and angiitis. Definition of the syndrome has altered over time, but according to a recent classification scheme it has been included in the same group as Wegener's granulomatosis and microscopic polyangiitis, both of which are strongly associated with anti-neutrophil cytoplasmic antibodies (ANCA), but whether Churg-Strauss truly belongs to this group is still yet to be definitively determined.
A multisystem disorder -- like all the vasculitides -- the manifestations of Churg-Strauss can be protean, but the most commonly symptomatic organ is the lungs, and eosinophilic asthma is considered a hallmark of the disease.
The American College or Rheumatology included "non-fixed pulmonary infiltrates on chest radiography" in 1990 as one of the six criteria for Churg-Strauss (4+/6 has a sensitivity of 85% and specificity of 99.7%)...
... but not every diagnosis scheme for Churg-Strauss includes imaging, and some of the alternate schemes claim a similar or better sensitivity and specificity.
This isn't totally surprising, because the radiographic findings in Churg-Strauss are incredibly nonspecific. The most commonly correlated findings on Chest CT are nonspecific groundglass opacities, usually with a peripheral distribution... or centrilobular nodules and tree-in-bud opacities, almost equally as nonspecific. Both the upper and lower lung zone can be affected, but the symptoms are usually more mild than with Wegener's granulomatosis. Cavitary nodules are reported as rare in Churg-Strauss, and if cavitary nodules are present, another etiology (e.g. Wegener's) should be entertained.
A multisystem disorder -- like all the vasculitides -- the manifestations of Churg-Strauss can be protean, but the most commonly symptomatic organ is the lungs, and eosinophilic asthma is considered a hallmark of the disease.
The American College or Rheumatology included "non-fixed pulmonary infiltrates on chest radiography" in 1990 as one of the six criteria for Churg-Strauss (4+/6 has a sensitivity of 85% and specificity of 99.7%)...
1) asthma
2) eosinophilia of greater than 10% of the peripheral WBC count
3) mononeuropathy or polyneuropathy
4) non-fixed non-fixed pulmonary infiltrates on chest radiography
5) paranasal sinus abnormalities
6) biopsy containing a blood vessle with extravascular eosinophils
... but not every diagnosis scheme for Churg-Strauss includes imaging, and some of the alternate schemes claim a similar or better sensitivity and specificity.
This isn't totally surprising, because the radiographic findings in Churg-Strauss are incredibly nonspecific. The most commonly correlated findings on Chest CT are nonspecific groundglass opacities, usually with a peripheral distribution... or centrilobular nodules and tree-in-bud opacities, almost equally as nonspecific. Both the upper and lower lung zone can be affected, but the symptoms are usually more mild than with Wegener's granulomatosis. Cavitary nodules are reported as rare in Churg-Strauss, and if cavitary nodules are present, another etiology (e.g. Wegener's) should be entertained.
Saturday, November 10, 2012
The Bugs Burst Out -- Mycotic Aneurysm
Aneurysms arise when the arterial wall is weakened and the arterial pressure overcomes the tensile strength of the wall (see "Laplace's theorem" post on 11/7/12). This wall weakness and the formation of an aneurysm is most often a combination of atherosclerosis, aging (cystic medial necrosis), and hypertension...
...but this isn't the only way to weaken the arterial wall. A mycotic aneurysm can also arise if an infectious process begins to weaken the wall. Although this a much less common event than a "bland" aneurysm, its prognosis is different and its treatment can be more tricky.
There are four main ways that an infectious aneurysm can form:
1. Septic emboli of cardiac origin ("Mycotic aneurysm") lodging in the lumen or vasa vasorum of peripheral arteries. These "mycotic" aneurysms (more likely bacterial... not fungal) have been recorded as occuring in virtually every artery, although they more commonly occur in the aorta, intracranial, superior mesenteric, and femoral arteries. The continued refinement of antibacterial therapy and the ability to replace infected cardiac valves have lead to a decrease in this etiology. Because the etiology is partly embolic, these aneurysms tend to form at arterial branch points and occlusions.
2. Microbial arteritis with aneurysm formation: With the decline of the prevalence of intracardiac vegetations, this etiology -- although rare -- has been becoming relatively more common. If the intima is interrupted (e.g. atherosclerosis), then bacteria have the capacity to penetrate into the arterial wall. If the infection takes hold, a pseudoaneurysm can result. Compatible with atherosclerosis, the aorta is the most common site for this process. Immunsuppresion, hemodialysis, and radiation arteritis are considered risk factors. It has been noted that the diseased aorta is more vulnerable to Salmonella spp.
3. Infection of a pre-existing aneurysm: Although colonization of aortic aneurysm walls has been shown to not be rare (~15%), whether infection of a pre-existing aneurysm is a significant mechanism for development of an infectious/inflammatory aneurysm is still controversial.
4. Post-traumatic infected pseudoaneurysm: These are reported as being more common in IVDUs and an increasing incidence of this etiology may also be partly due to increased numbers of percutaneous interventional therapies.
Signs of an infected central arterial aneurysm can be subtle. Elevated WBCs, ESR are sensitive, but not specific. Positive blood cultures in a patient with an aneurysm increase the specificity, but are not as sensitive (50%), so negative blood cultures alone are not enough to rule out the diagnosis.
With the lack of a definitive lab test, imaging becomes vital.
Although U/S is the usual screening tool for abdominal aortic aneurym, it is unable to differentiate between an infectious or a bland aneurysm.
CTA, however, provides the information necessary to help with a diagnosis.
Although U/S is the usual screening tool for abdominal aortic aneurym, it is unable to differentiate between an infectious or a bland aneurysm.
CTA, however, provides the information necessary to help with a diagnosis.
Friday, November 9, 2012
The SA Node Artery
Continuing down the RCA... right after the conus artery, a small branch splits off to supply the sinoatrial (SA) node, the aptly named SA node artery.
...Well, it sometimes (~55-65%) comes off the right coronary artery... the rest of the time it arises from the LCx (30-45%).
A smaller % (<10%) of patients have a dual blood supply.
But regardless of which circulation it arises from, it can be recognized by its course toward the SVC.
...Well, it sometimes (~55-65%) comes off the right coronary artery... the rest of the time it arises from the LCx (30-45%).
A smaller % (<10%) of patients have a dual blood supply.
But regardless of which circulation it arises from, it can be recognized by its course toward the SVC.
Thursday, November 8, 2012
Maximum fluid intensity -- bSSFP MRA
An MRI sequence that's not always recognized as an angiogram is the balanced steady-state free precession (bSSFP sequence... a.k.a. "trueFISP" or "b-TFE" or "FIESTA").
But what is a balanced steady-state free precession sequence?
Well... first of all, what is a steady-state free precession sequence?
1) Steady-state sequence and "unspoiled" GRE
The steady-state sequence is inherently a gradient echo technique... but it's considered "T2/T1 weighted" (which results in it having the highest signal intensity of all sequences)... so what exactly does this mean?
First... how is it T2-weighted?
Unspoiled gradient echo relies on a short TR (time to repetition). If the TR is shorter than the T2 relaxation time, residual coherent transverse magnetization builds with each RF pulse (T2-weighted imaging relies on transverse magnetization for its effects). Remember that for a time of flight sequence (see posts from 10/14 and 10/17), repetitive short TR radiofrequency pulses do not allow much recovery of longitudinal magnetization, suppressing the the T1 signal and allowing for flow-related enhancement. The situation is similar here, with quick, repetitive RF pulses, building up coherent transverse magnetization
 |
| Example of an axial b-TFE image |
But what is a balanced steady-state free precession sequence?
Well... first of all, what is a steady-state free precession sequence?
1) Steady-state sequence and "unspoiled" GRE
The steady-state sequence is inherently a gradient echo technique... but it's considered "T2/T1 weighted" (which results in it having the highest signal intensity of all sequences)... so what exactly does this mean?
First... how is it T2-weighted?
Unspoiled gradient echo relies on a short TR (time to repetition). If the TR is shorter than the T2 relaxation time, residual coherent transverse magnetization builds with each RF pulse (T2-weighted imaging relies on transverse magnetization for its effects). Remember that for a time of flight sequence (see posts from 10/14 and 10/17), repetitive short TR radiofrequency pulses do not allow much recovery of longitudinal magnetization, suppressing the the T1 signal and allowing for flow-related enhancement. The situation is similar here, with quick, repetitive RF pulses, building up coherent transverse magnetization
Wednesday, November 7, 2012
Under Tension -- Laplace's Theorem
The tangential tension on a blood vessel is thought of as obeying Laplace's theorem -- tension is a product of the pressure on the wall and the radius of the vessel.
This formula is most appropriate for measuring force per unit tube length. For a more generalized case of tangential stress against the wall at a point, the wall thickness also plays an important role (as would seem intuituve)
Tangential tension = Pressure x radius
T = Pr
This formula is most appropriate for measuring force per unit tube length. For a more generalized case of tangential stress against the wall at a point, the wall thickness also plays an important role (as would seem intuituve)
Tangential
stress = Pressure x (radius/wall thickness)
t = P (r / w)
Tuesday, November 6, 2012
Shear-activated nanotherapeutic particles (SA-NTs)
...speaking of shear stress (11/5/12). The NEJM recently put out an article highlighting research in "Shear Activated Nanotherapeutic Particles" (SA-NTs).
To condense the article... the idea is that since flow dynamics are often altered in areas of vascular pathology (see the idea of "flow separation" in yesterday's post), this microenvironment could be a way to target drug therapy. If nanoparticles could be engineered to release fibrinolytics in areas of high shear stress (e.g. areas of stenosis), then this highly targeted t-PA drug delivery could avoid the risks of systemic t-PA. According to the article, SA-NT bound with t-PA offers the same therapeutic effect as systemic t-PA at 1/100 the dose.
An image from the article demonstrates the principle (below)
... but since at a bifurcation of blood vessels, there are natural, nonpathologic regions of high shear stress, this raises the question why SA-NTs don't dissociate on their way to the stenosis. According to the article, the SA-NTs are only released by "pathologically high" shear stress.
Although a promising idea, the article stresses that there is still much research to be done before SA-NTs could be used clinically.
---
1. Wootton DM and Alevriadou BR. "The Shear Stress of Busting Blood Clots" N Engl J Med 2012; 367:1361-136
To condense the article... the idea is that since flow dynamics are often altered in areas of vascular pathology (see the idea of "flow separation" in yesterday's post), this microenvironment could be a way to target drug therapy. If nanoparticles could be engineered to release fibrinolytics in areas of high shear stress (e.g. areas of stenosis), then this highly targeted t-PA drug delivery could avoid the risks of systemic t-PA. According to the article, SA-NT bound with t-PA offers the same therapeutic effect as systemic t-PA at 1/100 the dose.
An image from the article demonstrates the principle (below)
 |
| (from Ref 1) |
... but since at a bifurcation of blood vessels, there are natural, nonpathologic regions of high shear stress, this raises the question why SA-NTs don't dissociate on their way to the stenosis. According to the article, the SA-NTs are only released by "pathologically high" shear stress.
Although a promising idea, the article stresses that there is still much research to be done before SA-NTs could be used clinically.
---
1. Wootton DM and Alevriadou BR. "The Shear Stress of Busting Blood Clots" N Engl J Med 2012; 367:1361-136
Monday, November 5, 2012
Hemodynamics -- Turbulence & flow separation
If only blood flow were as simple as concentric fluid layers sliding over each other in an ideal tube. The truth is much more turbulent.
Pouiselle's law (see the 10/28/2012 post) acts as if blood were a laminar fluid. In this situation, resistance is linear with respect to pressure and flow. But human blood vessels don't usually fufill the criteria necessay for the development of the nice, orderly parabolic flow of Pouiselle's law. Tortuosity and multiple branch points end up causing shear to the blood flow, and complex helical blood flow is often the result.
Furthermore, at a certain velocity -- the critical velocity -- the laminar model breaks down and the fluid takes on a life of its own. Eddies, vortices, and random velocity vectors form in the flow and become a separate, significant factor for resistance to the flow. When this occurs, flow is roughly proportional to √ (∆P).
So what affects how quickly a fluid becomes turbulent? Reynolds found that the fluid's
density (ρ) g/cm3
velocity (ν) cm/sec
diameter of the vessel (D) (cm), and
viscosity (μ) g/sec/cm2
were all factors, and related by:
Pouiselle's law (see the 10/28/2012 post) acts as if blood were a laminar fluid. In this situation, resistance is linear with respect to pressure and flow. But human blood vessels don't usually fufill the criteria necessay for the development of the nice, orderly parabolic flow of Pouiselle's law. Tortuosity and multiple branch points end up causing shear to the blood flow, and complex helical blood flow is often the result.
Furthermore, at a certain velocity -- the critical velocity -- the laminar model breaks down and the fluid takes on a life of its own. Eddies, vortices, and random velocity vectors form in the flow and become a separate, significant factor for resistance to the flow. When this occurs, flow is roughly proportional to √ (∆P).
So what affects how quickly a fluid becomes turbulent? Reynolds found that the fluid's
density (ρ) g/cm3
velocity (ν) cm/sec
diameter of the vessel (D) (cm), and
viscosity (μ) g/sec/cm2
 |
| Reynold's original 1883 apparatus from which he formulated the relationship of Reynold's number. |
Sunday, November 4, 2012
The Uterine Artery
Subselective catheterization of the uterine artery can be important in a wide range of applications, from uterine artery embolization (UAE), to embolic treatment of adenomyosis, or for control of postpartum uterine bleeding.
The uterine artery is usually a branch off the internal iliac artery (anterior division), and can be recognized by its characteristic location and its strikingly serpiginous distribution. It frequently anastomoses with the nearby ipsilateral ovarian artery (which originates more distantly from the aorta, just below the renal arteries (or occasionally from the renal arteries). This anastomosis can become significant in embolization procedures. Occasionally, the ovarian artery may supply a large portion of the uterus, leading to incomplete embolization. Nontarget embolization from the uterine artery to the ovary, leading to premature ovarian failure is also an important consideration.
The arterial anastomoses between the uterine artery and ovarian artery has been shown to be less than 500 microns normally, and use of embolization particles larger than this may help prevent nontarget embolization of the ipislateral ovary.
The uterine artery is usually a branch off the internal iliac artery (anterior division), and can be recognized by its characteristic location and its strikingly serpiginous distribution. It frequently anastomoses with the nearby ipsilateral ovarian artery (which originates more distantly from the aorta, just below the renal arteries (or occasionally from the renal arteries). This anastomosis can become significant in embolization procedures. Occasionally, the ovarian artery may supply a large portion of the uterus, leading to incomplete embolization. Nontarget embolization from the uterine artery to the ovary, leading to premature ovarian failure is also an important consideration.
The arterial anastomoses between the uterine artery and ovarian artery has been shown to be less than 500 microns normally, and use of embolization particles larger than this may help prevent nontarget embolization of the ipislateral ovary.
Saturday, November 3, 2012
Eustachian Valve
At the other end from the crista terminalis is the eustachian valve. This structure is also known as "the valve of the IVC" and occurs
at the IVC/RA junction. It is theorized to help direct blood flow from
the IVC to the foramen ovale in the fetus.
The Eustachian valve occasionally has remnants thick enough to be detected on cardiac CT, ECHO, and sometimes, even with angiography.
 |
| The red transparent oval shows how the intact eustachian valve in the fetus would help direct blood flow through the foramen ovale, bypassing the right-sided circulation |
The Eustachian valve occasionally has remnants thick enough to be detected on cardiac CT, ECHO, and sometimes, even with angiography.
Friday, November 2, 2012
Crista Terminalis
Thursday, November 1, 2012
The Two Branches of the External Iliac Artery
Unlike the internal iliac artery, with its multiple branches to supply the muscles and organs of the pelvis, the external iliac artery is more or less a straight shot through the pelvis to supply the lower extremity.
More or less... there are actually two branches off the external iliac right near the inguinal ring -- one more recognized, and the other... less so.
First... before enumerating the branches... a quick review. What actually demarcates the external iliac artery? Well... the origin is obvious. It begins at the branch point of the common iliac arteries into the internal and external arteries. But where's the end point? As soon as the external iliac passes beneath the inguinal ligament, through the femoral canal, it becomes the femoral artery... But in the angiography suite, the inguinal ligament is not likely to be visible during a DSA run, so the branches near the inguinal ring act as a surrogate marker for the transition.
So... these two branches:
More or less... there are actually two branches off the external iliac right near the inguinal ring -- one more recognized, and the other... less so.
First... before enumerating the branches... a quick review. What actually demarcates the external iliac artery? Well... the origin is obvious. It begins at the branch point of the common iliac arteries into the internal and external arteries. But where's the end point? As soon as the external iliac passes beneath the inguinal ligament, through the femoral canal, it becomes the femoral artery... But in the angiography suite, the inguinal ligament is not likely to be visible during a DSA run, so the branches near the inguinal ring act as a surrogate marker for the transition.
So... these two branches:
1. The inferior epigastric artery
2. The deep iliac circumflex artery
Wednesday, October 31, 2012
Embolizing the Thoracic Duct
a.k.a. "misadventures in thoracic surgery"
Rarely, the thoracic duct is damaged during thoracic surgery, and can be detected as a left-sided chylous pleural effusion (chylopericardium has also been reported). The traditional treatment for thoracic duct injury with left chylothorax has been clipping the thoracic duct followed by pleurodesis, but in 1998 percutaneous access of the thoracic duct was first attempted, and a 45-71% success rate of percutaneous embolization has lead some to try it as a first pass to avoid surgery.
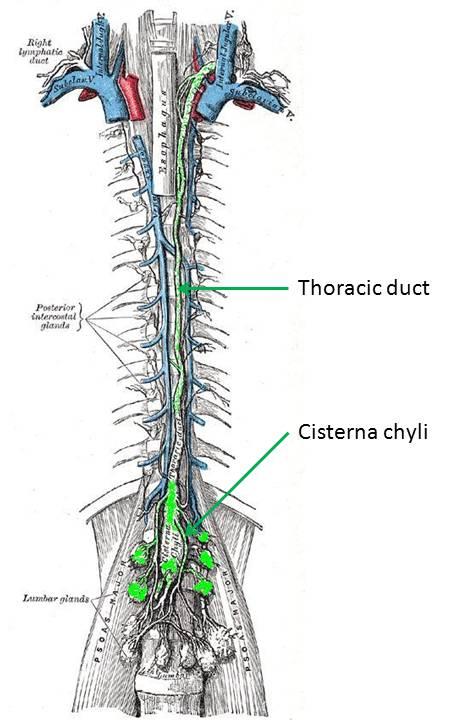 Transection of the thoracic duct is not the only way to cause a
chylothorax, however. It has been noted that compression or stenosis of
the thoracic duct leads to the development of small, fragile
collaterals running in parallel. These thin collaterals are easily
damaged and can rupture into the pleural space.
Transection of the thoracic duct is not the only way to cause a
chylothorax, however. It has been noted that compression or stenosis of
the thoracic duct leads to the development of small, fragile
collaterals running in parallel. These thin collaterals are easily
damaged and can rupture into the pleural space.
First, a little bit about the thoracic duct... The lymphatics begin as end-bulbs (lacteals in the intestine) and merge into progressively larger lymphatic channels. The lymph moves centrally due to the action of one-way valves, spontaneous contraction of lymphatic channels, and muscular or organ activity. These channels eventually converge around the L1-L2 vertebra. If the confluence is centralized and fusiform, it's termed a cisterna chyli. The thoracic duct arises from this level and rises between the esophagus and aorta, to empty into the left internal jugular or left subclavian vein... thus returning the lymph to the vascular blood stream. The thoracic duct can have multiple or duplicated channels within it. Lymph at the periphery is clear and colorless, but lymph originating at the intestine (from the lacteals) is often cloudy since it is a suspension containing fat (about 60-70% of fat makes its way to the bloodstream through the lymphatic system).
So how do you access the thoracic duct? Well... that's the trick. It would seem tempting to try to cannulate it in a retrograde manner through its cephalad venous confluence, but this is not only very technically difficult, but if there is a complete transection of the skinny thoracic duct, then you also won't be able to embolize the outflow channel.
So instead, you have to go antegrade... but how do you find the upstream entrance to the thoracic duct? The trick is to perform a pedal lymphangiogram first as a road map. First lymphazurin is injected into the subcutaneous soft tissues (at the interdigital spaces of the first, second, and third toes). A small incision is then made and the colored lymphazurin ("lymph" + "azure") highlights the foot lymphatic channels in the subcutaneous tissues. A lymphatic channel is then selected and cannulated with a 30 gauge catheter, after which lipiodol is slowly infused (below). Multiple lymphatic channels can be cannulated to try to increase the amount of contrast in the lymphatic system.
Rarely, the thoracic duct is damaged during thoracic surgery, and can be detected as a left-sided chylous pleural effusion (chylopericardium has also been reported). The traditional treatment for thoracic duct injury with left chylothorax has been clipping the thoracic duct followed by pleurodesis, but in 1998 percutaneous access of the thoracic duct was first attempted, and a 45-71% success rate of percutaneous embolization has lead some to try it as a first pass to avoid surgery.
 Transection of the thoracic duct is not the only way to cause a
chylothorax, however. It has been noted that compression or stenosis of
the thoracic duct leads to the development of small, fragile
collaterals running in parallel. These thin collaterals are easily
damaged and can rupture into the pleural space.
Transection of the thoracic duct is not the only way to cause a
chylothorax, however. It has been noted that compression or stenosis of
the thoracic duct leads to the development of small, fragile
collaterals running in parallel. These thin collaterals are easily
damaged and can rupture into the pleural space.First, a little bit about the thoracic duct... The lymphatics begin as end-bulbs (lacteals in the intestine) and merge into progressively larger lymphatic channels. The lymph moves centrally due to the action of one-way valves, spontaneous contraction of lymphatic channels, and muscular or organ activity. These channels eventually converge around the L1-L2 vertebra. If the confluence is centralized and fusiform, it's termed a cisterna chyli. The thoracic duct arises from this level and rises between the esophagus and aorta, to empty into the left internal jugular or left subclavian vein... thus returning the lymph to the vascular blood stream. The thoracic duct can have multiple or duplicated channels within it. Lymph at the periphery is clear and colorless, but lymph originating at the intestine (from the lacteals) is often cloudy since it is a suspension containing fat (about 60-70% of fat makes its way to the bloodstream through the lymphatic system).
So how do you access the thoracic duct? Well... that's the trick. It would seem tempting to try to cannulate it in a retrograde manner through its cephalad venous confluence, but this is not only very technically difficult, but if there is a complete transection of the skinny thoracic duct, then you also won't be able to embolize the outflow channel.
So instead, you have to go antegrade... but how do you find the upstream entrance to the thoracic duct? The trick is to perform a pedal lymphangiogram first as a road map. First lymphazurin is injected into the subcutaneous soft tissues (at the interdigital spaces of the first, second, and third toes). A small incision is then made and the colored lymphazurin ("lymph" + "azure") highlights the foot lymphatic channels in the subcutaneous tissues. A lymphatic channel is then selected and cannulated with a 30 gauge catheter, after which lipiodol is slowly infused (below). Multiple lymphatic channels can be cannulated to try to increase the amount of contrast in the lymphatic system.
 |
| Lipiodol contrast extending up the lymphatics of the lower extremity toward the pelvis, and from there to the abdomen and cisterna chyli. |
Tuesday, October 30, 2012
Hemodynamics: Collateral Flow
The concept of collateral flow in the vascular circuit is not too difficult to appreciate and has a wide variety of applications in arterial physiology. The concept is similar to flow in electrical circuits. If in parallel, vascular resistances are additive as reciprocals, that is...
For a vascular circuit like the one above, this means that flow through all resistances (Rt) is greater than flow through any one resistance. This makes intuitive sense.
The resistance of any one tube can be estimated by the Pouiselle law (see post from 10/28/12), so:
1/Rt = 1/R1 + 1/R2 + 1/R3 + ... + 1/Rn
For a vascular circuit like the one above, this means that flow through all resistances (Rt) is greater than flow through any one resistance. This makes intuitive sense.
The resistance of any one tube can be estimated by the Pouiselle law (see post from 10/28/12), so:
laminar resistance in a tube (R) = (8 η L) / (π r^4 )
Monday, October 29, 2012
May-Thurner Syndrome
The left common iliac vein is in a tight spot, caught as it is between the right common iliac artery and the lumbar/sacral spine. Like the "Nutcracker" syndrome (see post "10/3/2012"), some people are more prone to AP compression of the abdomen and pelvis for whatever reason. Oddly enough, the demographics for May-Thurner syndrome, like the Nutcracker Syndome is mostly female (3:1), younger (10s-30s), and it also occurs more frequently in patients who have had multiple pregnancies.
This relative narrowing at the downstream common iliac vein is theorized to lead to an increase in left-sided deep venous thrombus. May-Thurner is really just a subset of central venous occlusion syndromes... and as with central venous occlusion, lower extremity venous stasis is not appreciably helped by change in position (such as with stasis from valvular incompetence). Nor would the Doppler waveform change appreciably with Valsalva.... nor would it resolve with compression stockings or leg exercise.
Compression of the left iliac vein can result in a rage of presentations: from asymptomatic (with a pressure gradient across the compression of < 2 mmHg), so the development of venous "spurs" (described below), to the development of extensive pelvic collaterals with or without pelvic and lower extremity thrombosis.(May-Thurner syndrome).
This relative narrowing at the downstream common iliac vein is theorized to lead to an increase in left-sided deep venous thrombus. May-Thurner is really just a subset of central venous occlusion syndromes... and as with central venous occlusion, lower extremity venous stasis is not appreciably helped by change in position (such as with stasis from valvular incompetence). Nor would the Doppler waveform change appreciably with Valsalva.... nor would it resolve with compression stockings or leg exercise.
Compression of the left iliac vein can result in a rage of presentations: from asymptomatic (with a pressure gradient across the compression of < 2 mmHg), so the development of venous "spurs" (described below), to the development of extensive pelvic collaterals with or without pelvic and lower extremity thrombosis.(May-Thurner syndrome).
 |
| Stenosis at the characteristic May-Thurner location. The left iliac vein is also smaller than its counterpart, presumably due to slow flow. |
Sunday, October 28, 2012
Hemodynamics - Pouiselle's Law
Although a very simplified version of blood flow through a vessel -- Pouiselle's model for flow in a cylindrical tube is useful for arranging the relationships between variables in the hemodynamic circulation. In particular, the incredible importance of blood flow on the radius of the vessel (directly proportional to the fourth power), which is conceptually applicable in all sorts of clinical situations (vasodilators/vasocontrictors/atherosclerosis/etc.)
Saturday, October 27, 2012
Pelvic Artery Ectasia/Aneurysm
Although isolated aneurysms of the distal abdomen are not uncommon, and aneurysms extending into the iliacs are not uncommon... an isolated aneurysm of the common iliac artery is rare... very rare (about 0.03%)
But ectasia of the larger pelvic arteries is not rare at all. So what is normal? what is ectasia? what is aneurysm? At some point you have to mention that an artery is larger than normal, but not really aneurysmal... and then the patient may get follow-up studies to follow its size. Where do you make that cut-off between "normal" and "follow"? Is it just arbitrary?
Different literature sources (ultrasound, cardiology, interventional radiology, vascular surgery) do not agree on an exact number... for instance, in different literature sources, the mean normal common iliac artery size ranged between 12 - 15 mm ... but since the absolute difference between these values is small... and since the range is not too wide... a lot of the variation in the literature can be swallowed up in differences in interobserver measurement technique (up to 3mm with ultrasound?) or differences in modality. In one study, ultrasound underestimated normal-sized aortas relative to CT, and overestimated large aneurysms, and it's not really clear that either could be used as a gold standard.
.JPG) |
| "Max" is the maximum value of normal (approx 2 s.d.) |
Different literature sources (ultrasound, cardiology, interventional radiology, vascular surgery) do not agree on an exact number... for instance, in different literature sources, the mean normal common iliac artery size ranged between 12 - 15 mm ... but since the absolute difference between these values is small... and since the range is not too wide... a lot of the variation in the literature can be swallowed up in differences in interobserver measurement technique (up to 3mm with ultrasound?) or differences in modality. In one study, ultrasound underestimated normal-sized aortas relative to CT, and overestimated large aneurysms, and it's not really clear that either could be used as a gold standard.
Friday, October 26, 2012
Persistent Sciatic Artery
 |
| (Ref 1) |
As the embryo develops, it regresses to the level of the popliteal artery...
... and then eventually involutes completely as the iliofemoral system replaces the sciatic artery system. The only portions that remain are segments of the popliteal and fibular arteries... as well as portions of the inferior and superior gluteal arteries.
Rarely (0.025 - 0.05%), the sciatic artery persists into adult life. There is variability in the degree of how much it persists -- anywhere from complete persistence from the internal iliac artery to the poplitieal, to partial persistence with connection from the internal iliac artery through multiple collaterals. Angiographically, it should be suspected when there is enlargement of the internal iliac artery relative to the external, and an abnormal common femoral artery. It is reported as bilateral 25% of the time.
Thursday, October 25, 2012
Internal Iliac artery: Posterior Division: Superior Gluteal Artery
The external iliac artery in the pelvis is usually pretty straightforward -- more or less just a straight shot through pelvis, with a little inferior epigastric branch and deep circumflex iliac branch usually coming off.
The internal iliac artery ("hypogastric artery"), however, is a different matter. There is extensive variation of its numerous branches, which usually initially branch into two divisions anterior and posterior before branching out into numerous vessels to supply the pelvic muscles and organs. Because of the extensive variation, identification of these vessels is usually easier from looking at what they supply rather than from where they originate.
The posterior division of the internal iliac artery typically has three branches:
1. The iliolumbar artery usually arises most proximally, and can arise from the proximal iliac artery before the bifurcation into the anterior and posterior divisions. It courses superiorly, overlying the region of the sacroiliac joint.
2. The lateral sacral arteries are small and variable, and named for their position overlying the lateral sacrum. There are usually two, but they can be up to four in number. The anastomose with the median sacral artery and lateral sacral vessels.
3. The superior gluteal artery (SGA) is the largest artery of the posterior division, and courses posteriorly through the greater sciatic foramen, above the piriformis muscle. Its large size supplies the gluteal musculature (and piriformis). It is the most commonly injured pelvic artery in a pelvic fracture, with shearing against the bony portion of the greater sciatic foramen. (The next most common are the internal pudendal artery and the obturator artery). The SGA forms multiple collaterals with other pelvic arteries, including the inferior gluteal artery, medial circumflex, and lateral femoral circumflex.
The internal iliac artery ("hypogastric artery"), however, is a different matter. There is extensive variation of its numerous branches, which usually initially branch into two divisions anterior and posterior before branching out into numerous vessels to supply the pelvic muscles and organs. Because of the extensive variation, identification of these vessels is usually easier from looking at what they supply rather than from where they originate.
The posterior division of the internal iliac artery typically has three branches:
1. The iliolumbar artery
2. The lateral sacral arteries
3 The superior gluteal artery
1. The iliolumbar artery usually arises most proximally, and can arise from the proximal iliac artery before the bifurcation into the anterior and posterior divisions. It courses superiorly, overlying the region of the sacroiliac joint.
2. The lateral sacral arteries are small and variable, and named for their position overlying the lateral sacrum. There are usually two, but they can be up to four in number. The anastomose with the median sacral artery and lateral sacral vessels.
3. The superior gluteal artery (SGA) is the largest artery of the posterior division, and courses posteriorly through the greater sciatic foramen, above the piriformis muscle. Its large size supplies the gluteal musculature (and piriformis). It is the most commonly injured pelvic artery in a pelvic fracture, with shearing against the bony portion of the greater sciatic foramen. (The next most common are the internal pudendal artery and the obturator artery). The SGA forms multiple collaterals with other pelvic arteries, including the inferior gluteal artery, medial circumflex, and lateral femoral circumflex.
Wednesday, October 24, 2012
How to Survive an Air Embolism
The best way to survive a venous air embolism is to avoid getting one in the first place.
Symptomatic air embolism during the placement of a central venous catheter (as discovered by a radiolucency over the heart during fluoro) is a dangerous but fortunately uncommon event. There is risk for entry of air into the blood stream with placement of an IJ or subclavian central venous catheter, due to the negative intrathoracic pressure. The traditional patient positioning for placement of a line to avoid entry of air is Trendelenberg and with the patient performing a Valsalva maneuver (to increase intrathoracic pressure).
Placement of a central line is not the only possible etiology for a venous air embolism -- they've also been reported with detachment of the IV tubing from the catheter hub, failure to close the hub, a fractured catheter, or air entering a persistent subcutaneous tunnel after catheter removal. And, of course, it's also possible to introduce air into the venous system with power injection of contrast in CT.
Many small venous air emboli are asymptomatic and the incidence of small venous air emboli may be higher than currently thought since most are not detected.... but what if enough air is introduced into the right heart to cause an "air lock" of the pulmonary outflow tract? What if the patient starts crashing?
Trendelenberg, left lateral decubitus (left side down), and oxygen.
The idea is to trap the air in the right atrium -- rather than in the PVOT -- by putting the right atrium most superiorly -- swing the vena cava up. It seems to make mechanical sense... I doubt this maneuver is ever going to make it to a randomized controlled trial.
The use of oxygen is two-fold in that it helps keep blood oxygen levels up, but it's also theorized to decrease the size of the embolism itself by causing nitrogen to diffuse out of the air bubbles. There have been case reports of attempting to introduce a catheter into the right heart to suck out the air, but it's debatable if this is really effective. If a catheter is in place, it may be worth a shot, but if not, it's doubtful if introduction of a new catheter would be worthwhile.
and of course, if this doesn't work...................... then start CPR.
---
1. Vesely TM. "Air Embolism during Insertion of Central Venous Catheters" J Vasc Interv Radiol 2001; 12:1291–1295
2. ACR Manual on Contrast Media, 8th ed (2012)
Symptomatic air embolism during the placement of a central venous catheter (as discovered by a radiolucency over the heart during fluoro) is a dangerous but fortunately uncommon event. There is risk for entry of air into the blood stream with placement of an IJ or subclavian central venous catheter, due to the negative intrathoracic pressure. The traditional patient positioning for placement of a line to avoid entry of air is Trendelenberg and with the patient performing a Valsalva maneuver (to increase intrathoracic pressure).
Placement of a central line is not the only possible etiology for a venous air embolism -- they've also been reported with detachment of the IV tubing from the catheter hub, failure to close the hub, a fractured catheter, or air entering a persistent subcutaneous tunnel after catheter removal. And, of course, it's also possible to introduce air into the venous system with power injection of contrast in CT.
Many small venous air emboli are asymptomatic and the incidence of small venous air emboli may be higher than currently thought since most are not detected.... but what if enough air is introduced into the right heart to cause an "air lock" of the pulmonary outflow tract? What if the patient starts crashing?
Trendelenberg, left lateral decubitus (left side down), and oxygen.
The idea is to trap the air in the right atrium -- rather than in the PVOT -- by putting the right atrium most superiorly -- swing the vena cava up. It seems to make mechanical sense... I doubt this maneuver is ever going to make it to a randomized controlled trial.
The use of oxygen is two-fold in that it helps keep blood oxygen levels up, but it's also theorized to decrease the size of the embolism itself by causing nitrogen to diffuse out of the air bubbles. There have been case reports of attempting to introduce a catheter into the right heart to suck out the air, but it's debatable if this is really effective. If a catheter is in place, it may be worth a shot, but if not, it's doubtful if introduction of a new catheter would be worthwhile.
and of course, if this doesn't work...................... then start CPR.
---
1. Vesely TM. "Air Embolism during Insertion of Central Venous Catheters" J Vasc Interv Radiol 2001; 12:1291–1295
2. ACR Manual on Contrast Media, 8th ed (2012)
Tuesday, October 23, 2012
The Vasculitides: Polyarteritis Nodosa
Polyarteritis nodosa (PAN) is a necrotizing vasculitis that affects medium and small arteries throughout the body. The inflammation causes fibrinoid necrosis of the arterial media and a cellular infiltrate predominantly of neutrophils and leukocytes. Aneurysms form as a result of this weakening of the arterial media.
Almost any organ or organ system can be affected, kidneys, liver, spleen, the heart, the lower extremities, the skin, the nervous system (e.g. mononeuritis multiplex)...but the classic presentation is numerous small aneurysms in the kidney (below) or liver. Constitutional symptoms (fever, weight loss) are also common, but obviously not very specific findings.
On angiography PAN presents with ectasia and narrowing of the medium-sized arteries, as well as with the previously mentioned microaneurysms. The visceral artery microaneurysms have been reported to occur more frequently at vascular bifurcations and have a tendency to bleed. Clearly, due to their small size, an angiographic technique with high spatial resolution is necessary for evaluation (selective DSA, well-timed CTA)
Almost any organ or organ system can be affected, kidneys, liver, spleen, the heart, the lower extremities, the skin, the nervous system (e.g. mononeuritis multiplex)...but the classic presentation is numerous small aneurysms in the kidney (below) or liver. Constitutional symptoms (fever, weight loss) are also common, but obviously not very specific findings.
 |
| "Right renal arteriogram shows one small aneurysm of an upper lobar branch (arrow) and irregular ectasia of the main renal artery (arrowhead)." (ref 1) |
On angiography PAN presents with ectasia and narrowing of the medium-sized arteries, as well as with the previously mentioned microaneurysms. The visceral artery microaneurysms have been reported to occur more frequently at vascular bifurcations and have a tendency to bleed. Clearly, due to their small size, an angiographic technique with high spatial resolution is necessary for evaluation (selective DSA, well-timed CTA)
 |
| 46 year-old male with known polyarteritis nodosa and new hemorrhage. (Courtesy Dr. D. Eschelman) |
Monday, October 22, 2012
Carotid Dissection on Ultrasound
When talking about the carotid arteries, the mind jumps almost immediately to ultrasound as a modality. Especially in the emergency setting -- what would be better than a fast, cheap, nonionizing test to rule out carotid dissection? You wouldn't have to radiate the patient's neck or wait hours for the MRI to finish up whatever lengthy cervical, thoracic, and lumbar scan is already in progress?
But is ultrasound effective as as a first line modality for carotid dissection? To feel confident ruling out carotid dissection in the ED, you'd need to be sure that it has a high sensitivity. One source claims that in patients with their first-ever carotid territory symptoms, "normal ultrasound findings in the cervical ICA allowed the reliable exclusion of an underlying [spontaneous internal carotid dissection] reflected by sensitivity and NPV values of 96% to 97%" (ref 3). In this group of patients, ultrasound was shown to have a slightly higher false positive rate than other modalities, and the authors conclude that if detected by ultrasound, another modality (e.g. MRA) should be used for verification before initiating therapy.
Importantly, in patients with "local symptoms and signs on the side of dissection (eg, headache, neck pain, Horner syndrome, and cranial nerve palsy" ultrasound is only 69-71% sensitive... so ultrasound is not appropriate for ruling out dissection in a patient with Horner syndrome only and no carotid territory ischemic signs.
The problem with using ultrasound as a screening modality in the ED is that old bugaboo -- operator dependence. Sensitivity and specificity are literally in the hands of your sonographer, so faith in their skills is paramount. Compounding that problem is that most spontaneous or traumatic carotid dissections begin in the distal ICA, which is a little trickier area to evaluate for a novice sonographer.
Furthermore, although ultrasound could be used to rule out carotid dissection in certain cirucmstances, it certainly isn't the definitve modality for evaluation. A spontaneous dissection usually stops at the skull base, but if it doesn't, it'll be tough to know by ultrasound. Maybe you have someone in your ED who can reliably perform transcranial Doppler of the ICA? I didn't think so. So if the dissection is solely in the petrous portion of the carotid or above, you'll miss it completely on ultrasound.
But is ultrasound effective as as a first line modality for carotid dissection? To feel confident ruling out carotid dissection in the ED, you'd need to be sure that it has a high sensitivity. One source claims that in patients with their first-ever carotid territory symptoms, "normal ultrasound findings in the cervical ICA allowed the reliable exclusion of an underlying [spontaneous internal carotid dissection] reflected by sensitivity and NPV values of 96% to 97%" (ref 3). In this group of patients, ultrasound was shown to have a slightly higher false positive rate than other modalities, and the authors conclude that if detected by ultrasound, another modality (e.g. MRA) should be used for verification before initiating therapy.
Importantly, in patients with "local symptoms and signs on the side of dissection (eg, headache, neck pain, Horner syndrome, and cranial nerve palsy" ultrasound is only 69-71% sensitive... so ultrasound is not appropriate for ruling out dissection in a patient with Horner syndrome only and no carotid territory ischemic signs.
The problem with using ultrasound as a screening modality in the ED is that old bugaboo -- operator dependence. Sensitivity and specificity are literally in the hands of your sonographer, so faith in their skills is paramount. Compounding that problem is that most spontaneous or traumatic carotid dissections begin in the distal ICA, which is a little trickier area to evaluate for a novice sonographer.
Furthermore, although ultrasound could be used to rule out carotid dissection in certain cirucmstances, it certainly isn't the definitve modality for evaluation. A spontaneous dissection usually stops at the skull base, but if it doesn't, it'll be tough to know by ultrasound. Maybe you have someone in your ED who can reliably perform transcranial Doppler of the ICA? I didn't think so. So if the dissection is solely in the petrous portion of the carotid or above, you'll miss it completely on ultrasound.
Sunday, October 21, 2012
Carotid Dissection on MRA
MRA is increasingly being employed for evaluation of carotid dissection (see 10/20/12 post), but although it has some significant advantages over traditional catheter-based angiography and CTA... it's not the ideal test in every circumstance.
The advantages?
- MRA with quick concurrent diffusion-weighted MRI sequences of the brain can evaluate infarction much better than CTA. (Cerebral infarction is documented in 42% of spontaneous carotid dissections)
- T1-weighted images of the neck are superior for evaluation of hematoma than catheter-based angiography (although CTA is also good for this).
- MRA avoids the 1% stroke risk of catheter-based angio (CTA also does this, of course)
- MRA avoids potential contrast-induced nephropathy (assuming that most patients being evaluated for dissection are young, renal failure and the risk of NSF are not as much of an issue)
- MRA avoids ionizing radiation
The disadvantages?
- The spatial resolution of MRA is not quite as high as that of CTA (perhaps Ablavar (10/19/12 post) would change this?), and concomitant evaluation of the vertebral arteries in the setting of trauma is better with CTA.
- Although the false lumen would be identified on the T1-weighted sequences, turbulent flow in an aneurysm can lead to signal drop out and underestimation of the size of the aneurysm on the MRA.
- MRA can be affected by metallic density artifact in then neck, rendering it dangerous or useless (CTA also has the problem of streak artifact with metallic densities). MRA is also affected by neck stablization hardware.
- MRA is slower than the other modalities.
The target population for carotid artery dissection is generally younger, and the lack of ionizing radiation in MRA is attractive, especially since these patients often receive multiple follow-up studies to evaluate the resolution/progression of the dissection and hematoma. Depending on your level of suspicion for acute infarction (in which diffusion-weighted sequences would be invaluable), CTA in the ED with MRA follow-up would seem a reasonable algorithm.
Recent reports claim that a T1 CUBE black blood sequence may offer improvement in detection of dissection compared to conventional axial T1 sequences... no problem, you just need that 3T scanner.
---
1. "Rutherford's Vascular Surgery" Cronenwett and Johnston. 7th ed. (2010)
2. Vertinskya AT, Schwartzb NE, Fischbeinc NJ, et al. "Comparison of Multidetector CT Angiography and MR Imaging of Cervical Artery Dissection" AJNR October 2008 29: 1753-1760
3. Edjlali M, Roca P, Rabrait C, et al. "3D Fast Spin-Echo T1 Black-Blood Imaging for the Diagnosis of Cervical Artery Dissection" Published online before print October 11, 2012, doi: 10.3174/ajnr.A3261
The advantages?
- MRA with quick concurrent diffusion-weighted MRI sequences of the brain can evaluate infarction much better than CTA. (Cerebral infarction is documented in 42% of spontaneous carotid dissections)
- T1-weighted images of the neck are superior for evaluation of hematoma than catheter-based angiography (although CTA is also good for this).
- MRA avoids the 1% stroke risk of catheter-based angio (CTA also does this, of course)
- MRA avoids potential contrast-induced nephropathy (assuming that most patients being evaluated for dissection are young, renal failure and the risk of NSF are not as much of an issue)
- MRA avoids ionizing radiation
 |
| Left internal carotid dissection with aneurysm (arrow). 3D TOF MIP MRA. |
The disadvantages?
- The spatial resolution of MRA is not quite as high as that of CTA (perhaps Ablavar (10/19/12 post) would change this?), and concomitant evaluation of the vertebral arteries in the setting of trauma is better with CTA.
- Although the false lumen would be identified on the T1-weighted sequences, turbulent flow in an aneurysm can lead to signal drop out and underestimation of the size of the aneurysm on the MRA.
- MRA can be affected by metallic density artifact in then neck, rendering it dangerous or useless (CTA also has the problem of streak artifact with metallic densities). MRA is also affected by neck stablization hardware.
- MRA is slower than the other modalities.
The target population for carotid artery dissection is generally younger, and the lack of ionizing radiation in MRA is attractive, especially since these patients often receive multiple follow-up studies to evaluate the resolution/progression of the dissection and hematoma. Depending on your level of suspicion for acute infarction (in which diffusion-weighted sequences would be invaluable), CTA in the ED with MRA follow-up would seem a reasonable algorithm.
Recent reports claim that a T1 CUBE black blood sequence may offer improvement in detection of dissection compared to conventional axial T1 sequences... no problem, you just need that 3T scanner.
---
1. "Rutherford's Vascular Surgery" Cronenwett and Johnston. 7th ed. (2010)
2. Vertinskya AT, Schwartzb NE, Fischbeinc NJ, et al. "Comparison of Multidetector CT Angiography and MR Imaging of Cervical Artery Dissection" AJNR October 2008 29: 1753-1760
3. Edjlali M, Roca P, Rabrait C, et al. "3D Fast Spin-Echo T1 Black-Blood Imaging for the Diagnosis of Cervical Artery Dissection" Published online before print October 11, 2012, doi: 10.3174/ajnr.A3261
Saturday, October 20, 2012
Carotid Dissection
A carotid dissection is just like a dissection in any other vessel in the body. Trauma or atherosclerosis causes a tear in the intima, blood enters into the space between intima and media, and then the blood dissects along that space causing stenosis or pseudoaneurysm. As it's sticking out there in the neck, the carotid is probably more liable to traumatic etiologies, than, say, the SMA. Extension of an aortic dissection can also cause a carotid dissection.
The dissection can re-enter the true lumen further down the artery, resulting in a false lumen. Thrombus can then exit out this second opening.
ICA (internal carotid artery) dissection has classically been described as irregular, originating 2-4 cm distal to the carotid bulb, and with a long tapering stenosis that usually ends before the ICA enters the petrous portion of the temporal bone.
Dissection of the carotid arteries or vertebral arteries, and the subsequent drop in cerebral flow, can be a cause of stroke, and this is a higher % etiology of stroke in young people, in whom the traditional risk factors are less likely. (Carotid dissection is the cause of 2% of ischemic strokes, but 10-20% of ischemic strokes in young or middle-aged persons). Carotid dissection can also cause neck and face pain, headache, amaurosis fugax, and Horner's syndrome.
The dissection can re-enter the true lumen further down the artery, resulting in a false lumen. Thrombus can then exit out this second opening.
ICA (internal carotid artery) dissection has classically been described as irregular, originating 2-4 cm distal to the carotid bulb, and with a long tapering stenosis that usually ends before the ICA enters the petrous portion of the temporal bone.
Dissection of the carotid arteries or vertebral arteries, and the subsequent drop in cerebral flow, can be a cause of stroke, and this is a higher % etiology of stroke in young people, in whom the traditional risk factors are less likely. (Carotid dissection is the cause of 2% of ischemic strokes, but 10-20% of ischemic strokes in young or middle-aged persons). Carotid dissection can also cause neck and face pain, headache, amaurosis fugax, and Horner's syndrome.
Friday, October 19, 2012
Ablavar
As a follow-up on MR angiography with contrast (10/18/12)... if the gadolinium ion is necessary for contrast, how can we deliver it to the blood stream? A salt solution of Gd-ions in buffered water?
No... that would be bad. Naked gadolinium (III) ions are toxic to the body, but can be safely transported through the bloodstream as a chelate. This actually works to the benefit of imaging, because, similar to principles in nuclear medicine, engineering the chelate can be useful in affecting its biodistribution. For example, using a chelate with a molecule which would be taken up by hepatocytes (Eovist), allows selective liver imaging.
So how could this be useful for MR angiography?
Since we want to image the vascular structures, it would be nice if we could keep the contrast in the vessels. For a conventional chelate, it pumps through the blood pool and is excreted out at a rate of 20% of cardiac output per pass through the kidneys. Multihance is generally used as an MRA contrast agent because a small % of it binds to the plasma proteins, which (hopefully most of the time) aren't excreted, thus prolonging its time in the blood pool.
A relatively-recently approved new chelate -- Ablavar -- improves on Multihance for contrast-enhanced MRA. Instead of a few % plasma protein binding, Ablavar binds at approximately 50%. Most extracellular agents have a half life of about 100 minutes... Ablavar lasts 15.5 hours.
Not only that, but the relaxivity of Ablavar is much higher than traditional extracellular agents -- about 4x higher.
Why is this a good thing? Well... contrast-enhanced MRA is very dependent on timing of the contrast bolus. Conventional agents "leak" out into the blood pool of the tissues and getting a good sequence is critically dependent on good bolus timing (and good centering of k-space)... but the extended acquisition time and increased relaxivity of Ablavar allows for longer steady-state acquisition times, and potentially submillimeter voxels. If you can get voxels this small without worsening of signal to noise, and thereby increasing the spatial resolution of the MR angiogram... then the overlay of veins in a steady-state MRA would not be a problem with multiplanar reformats. This is particularly promising for evaluation of the peripheral vasculature, which previously was difficult to assess due to "the inability to differentiate arteries from veins with a resolution of 1 mm3 that is typically acquired at standard-resolution first-pass MR angiography with gadolinium chelate contrast agent." Ablavar has the potential to open up MRA for evaluation of small vessels, such as those in the lower extremities and in the mesenteric circulation. Some early studies show that it may be as good as contrast-enhanced CT for the evaluation of many vascular pathologies.
Downsides? The spectre of NSF is always looming around gadolinium contrast agents, and though Ablavar's track record has no blemishes, it's still not totally proven. The fact that the increased relaxivity allows for smaller doses to be used is promising.
As a side note, although it can be used for a wide-variety of angiographic purposes, Ablavar is officially only FDA-approved for use in aorto-iliac disease, with the other uses being off-label. The suggest dose is 0.03 mmol/kg and the smaller dose that than of other extracellular contrast agents such as Multihance, result in a different bolus profile... and a decrease in the usual rate to about half has been found to be useful.
As another side note... the equilibrium phase for Ablavar imaging seems to work better with a decreased flip angle. Lowering the angle to around 20 degrees seems to provide a better image.
---
1. "Duke Review of MRI Principles: Case Review Series" Mangrum, et al. (2012).
2. Hadizadeh DR, Gieseke J, Lohmaier SH, et al. "Peripheral MR Angiography with Blood Pool Contrast Agent: Prospective Intraindividual Comparative Study of High-Spatial-Resolution Steady-State MR Angiography versus Standard-Resolution First-Pass MR Angiography and DSA" November 2008 Radiology, 249, 701-711.
3. Goyen M. "Gadofosveset-enhanced magnetic resonance angiography" Vascular Health and Risk Management 2008:4(1) 1–9
No... that would be bad. Naked gadolinium (III) ions are toxic to the body, but can be safely transported through the bloodstream as a chelate. This actually works to the benefit of imaging, because, similar to principles in nuclear medicine, engineering the chelate can be useful in affecting its biodistribution. For example, using a chelate with a molecule which would be taken up by hepatocytes (Eovist), allows selective liver imaging.
So how could this be useful for MR angiography?
Since we want to image the vascular structures, it would be nice if we could keep the contrast in the vessels. For a conventional chelate, it pumps through the blood pool and is excreted out at a rate of 20% of cardiac output per pass through the kidneys. Multihance is generally used as an MRA contrast agent because a small % of it binds to the plasma proteins, which (hopefully most of the time) aren't excreted, thus prolonging its time in the blood pool.
 |
| Gadofosveset ("Ablavar") |
Not only that, but the relaxivity of Ablavar is much higher than traditional extracellular agents -- about 4x higher.
Why is this a good thing? Well... contrast-enhanced MRA is very dependent on timing of the contrast bolus. Conventional agents "leak" out into the blood pool of the tissues and getting a good sequence is critically dependent on good bolus timing (and good centering of k-space)... but the extended acquisition time and increased relaxivity of Ablavar allows for longer steady-state acquisition times, and potentially submillimeter voxels. If you can get voxels this small without worsening of signal to noise, and thereby increasing the spatial resolution of the MR angiogram... then the overlay of veins in a steady-state MRA would not be a problem with multiplanar reformats. This is particularly promising for evaluation of the peripheral vasculature, which previously was difficult to assess due to "the inability to differentiate arteries from veins with a resolution of 1 mm3 that is typically acquired at standard-resolution first-pass MR angiography with gadolinium chelate contrast agent." Ablavar has the potential to open up MRA for evaluation of small vessels, such as those in the lower extremities and in the mesenteric circulation. Some early studies show that it may be as good as contrast-enhanced CT for the evaluation of many vascular pathologies.
 |
| Coronal image from an Ablavar-enhanced evaluation of the aorta |
Downsides? The spectre of NSF is always looming around gadolinium contrast agents, and though Ablavar's track record has no blemishes, it's still not totally proven. The fact that the increased relaxivity allows for smaller doses to be used is promising.
As a side note, although it can be used for a wide-variety of angiographic purposes, Ablavar is officially only FDA-approved for use in aorto-iliac disease, with the other uses being off-label. The suggest dose is 0.03 mmol/kg and the smaller dose that than of other extracellular contrast agents such as Multihance, result in a different bolus profile... and a decrease in the usual rate to about half has been found to be useful.
As another side note... the equilibrium phase for Ablavar imaging seems to work better with a decreased flip angle. Lowering the angle to around 20 degrees seems to provide a better image.
---
1. "Duke Review of MRI Principles: Case Review Series" Mangrum, et al. (2012).
2. Hadizadeh DR, Gieseke J, Lohmaier SH, et al. "Peripheral MR Angiography with Blood Pool Contrast Agent: Prospective Intraindividual Comparative Study of High-Spatial-Resolution Steady-State MR Angiography versus Standard-Resolution First-Pass MR Angiography and DSA" November 2008 Radiology, 249, 701-711.
3. Goyen M. "Gadofosveset-enhanced magnetic resonance angiography" Vascular Health and Risk Management 2008:4(1) 1–9
Subscribe to:
Posts (Atom)